Emerging Opportunities for Bispecific and Fusion Protein Therapeutics Beyond PD-1 Blockade
Summary
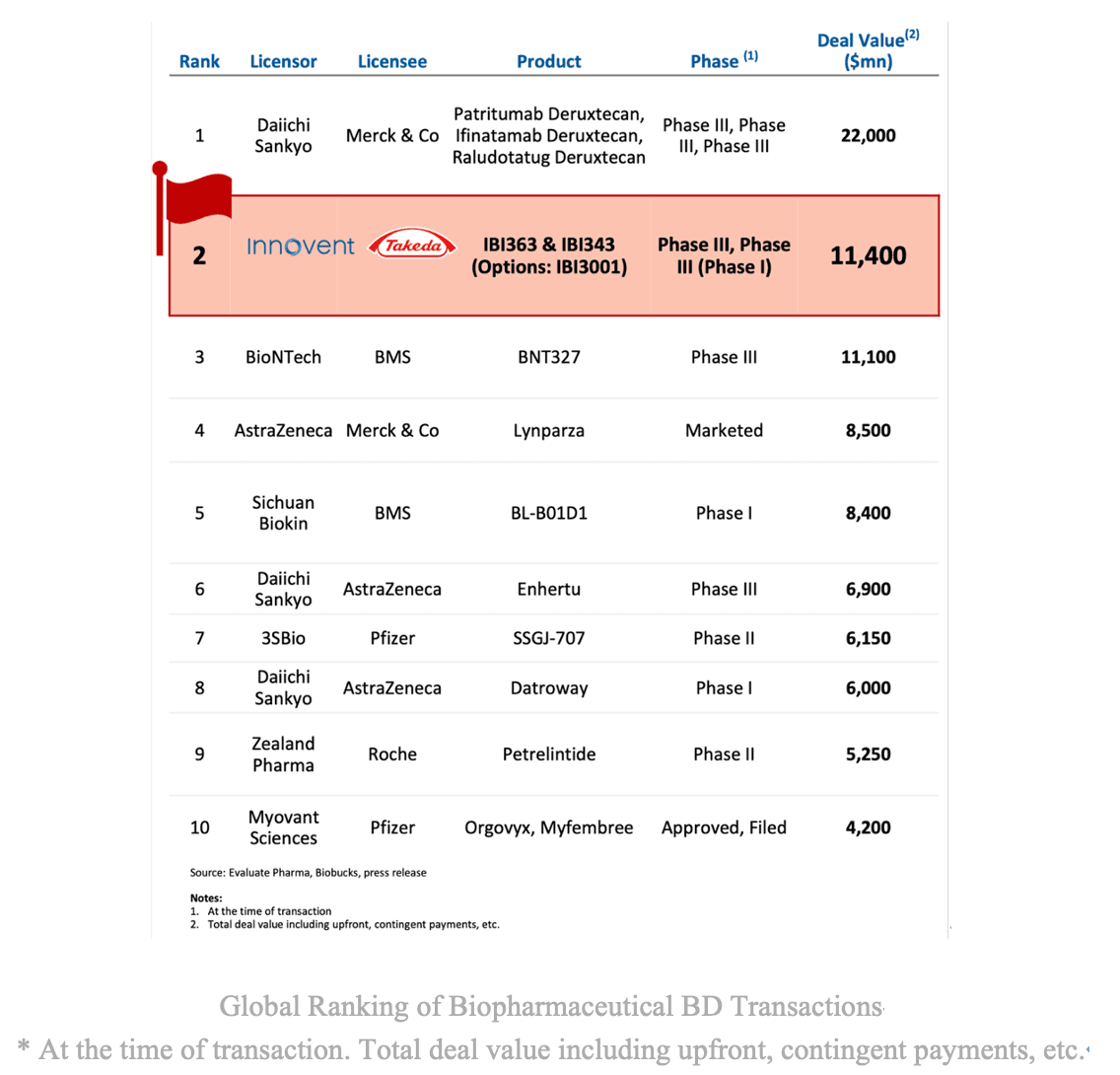
A record-breaking USD 11.4 billion transaction is reshaping the global immuno-oncology landscape. On Oct 22, 2025, Innovent Biologics and Takeda Pharmaceutical Company announced a historic global partnership centered on IBI363, a PD-1/IL-2α bispecific fusion protein --- marking not only the largest biopharma business development deal in China's history, but also a symbolic leap for innovative drug from China entering the global stage. This collaboration underscores that immune activation strategies beyond PD-1 blockade have become a new international focus in immunotherapy.
If you are interested in the emerging field of bispecific antibody and fusion protein therapeutics targeting PD-1, this analysis will guide you through the evolving opportunities beyond PD-1 blockade.
1. A Landmark Partnership: Innovent and Takeda
On Oct 22, 2025, Innovent Biologics and Takeda announced a landmark global strategic alliance covering 3 innovative oncology assets:
-
IBI363: A late-stage PD-1/IL-2α bispecific fusion protein showing breakthrough potential in non-small cell lung cancer (NSCLC) and colorectal cancer (CRC).
-
IBI343: An antibody--drug conjugate (ADC) targeting Claudin-18.2, developed for gastric and pancreatic cancers, both characterized by high mortality rates.
-
IBI3001: An early-stage bispecific ADC (EGFR/B7-H3), for which Takeda holds an exclusive global option right outside Greater China.
Under the agreement, Takeda will lead clinical development, manufacturing, and commercialization of IBI363 and IBI343 outside Greater China, while retaining an option to license IBI3001 globally. Both companies will co-develop and co-commercialize IBI363 for the U.S. and global markets, with Takeda holding 60% and Innovent 40%. Innovent will receive a USD 1.2 billion upfront payment (including USD 100 million strategic equity investment) and potential milestone payments totaling up to USD 11.4 billion, making it the largest BD deal in Chinese biopharma history.
With PD-1/PD-L1 inhibitors now widely adopted as backbone immunotherapies, global pharmaceutical companies are actively exploring next-generation approaches to overcome the efficacy ceiling, limited response rates, and resistance mechanisms of PD-1 blockade. Meanwhile, high-dose IL-2, once the first approved immunotherapy for renal cell carcinoma and melanoma, remains clinically restricted by severe vascular toxicity.
In this context, PD-1/IL-2α bispecific fusion proteins have emerged as one of the most promising solutions---offering precise immune reactivation through a synergistic mechanism that represents the transition from first-generation checkpoint inhibitors to precision immune modulation.
2. Mechanistic Insights: PD-1 and IL-2α
"PD-1 as the Brake, IL-2 as the Accelerator"
PD-1 (Programmed Death-1) is a classical immune checkpoint receptor expressed primarily on activated T cells, B cells, and subsets of myeloid cells. Its ligands, PD-L1 and PD-L2, are frequently expressed on tumor or immunosuppressive cells within the tumor microenvironment. When PD-1 engages its ligands, it suppresses T-cell proliferation, cytotoxic function, and cytokine secretion, effectively disabling immune attack against the tumor.
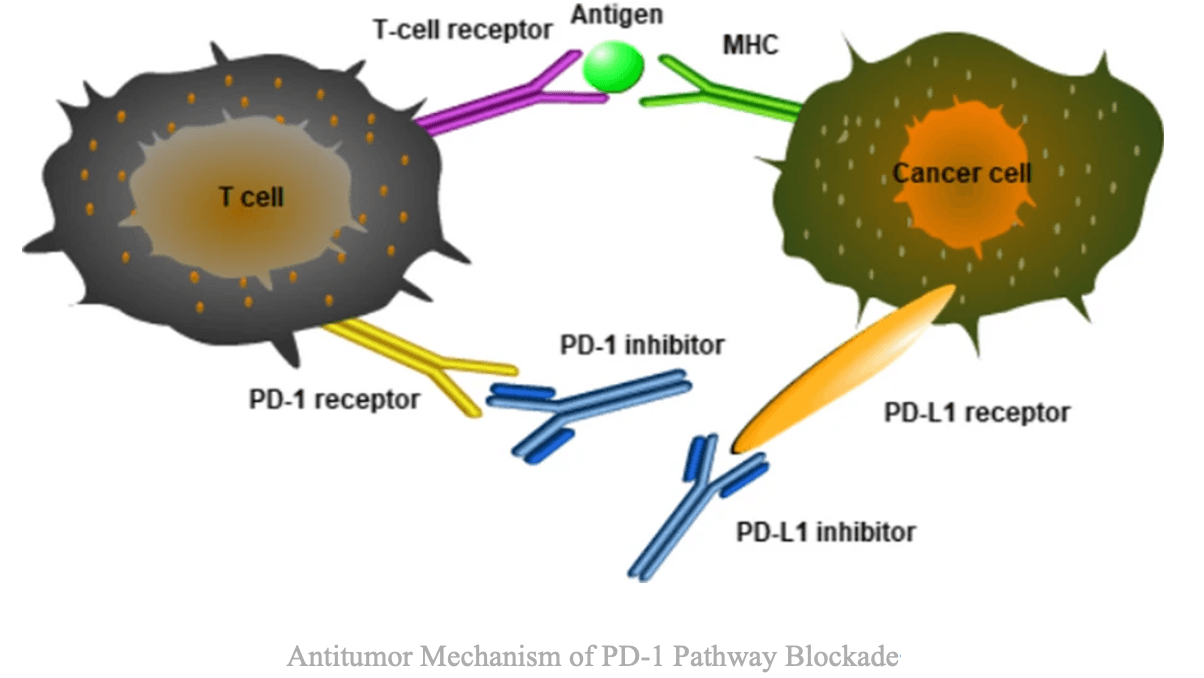
Conversely, IL-2 (Interleukin-2) acts as an immune accelerator. Secreted by activated T cells, IL-2 binds to the IL-2 receptor complex (comprising α, β, and γ subunits), stimulating T-cell and natural killer (NK) cell proliferation and cytotoxic activity. However, IL-2 also promotes the expansion of regulatory T cells (Tregs) and induces systemic toxicities, such as vascular leak syndrome, which limit its clinical use.
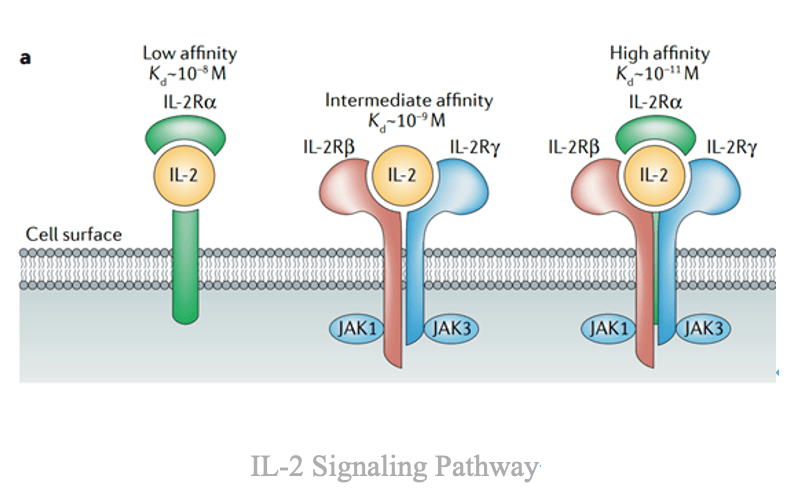
Thus, PD-1 serves as the brake, while IL-2 functions as the accelerator of the immune system.
The Limitations of PD-1 Monotherapy
Since the FDA approval of nivolumab in 2014, PD-1/PD-L1 inhibitors have become the cornerstone of modern immuno-oncology, producing durable responses across multiple solid tumors including melanoma, NSCLC, and renal cell carcinoma. However, limited response rates, resistance, and the inability to activate "cold tumors" have become increasingly evident.
To address these challenges, the next generation of immunotherapies seeks to both relieve immune inhibition and precisely activate effector immune cells---a key paradigm for innovation in immune-oncology.
The PD-1/IL-2α Synergy "1 + 1 > 2"
To overcome the ceiling of PD-1 monotherapy, "PD-1 Plus" combination strategies have been developed---pairing PD-1 blockade with targets such as CTLA-4, VEGF, or IL-15, achieving synergistic efficacy and broader patient applicability.
A PD-1/IL-2 bispecific fusion protein integrates both mechanisms---checkpoint release and cytokine activation---within a single molecule. This dual-functional construct simultaneously blocks PD-1/PD-L1 signaling ("releasing the brake") and activates IL-2 signaling ("stepping on the accelerator"), thereby driving strong and sustained effector T-cell activation while minimizing systemic toxicity.
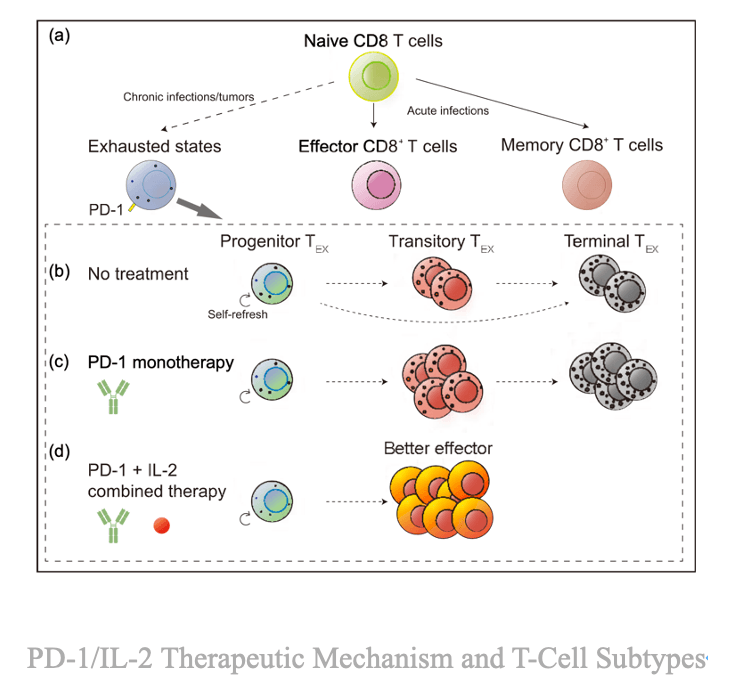
3. The Global PD-1/IL-2 Competitive Landscape
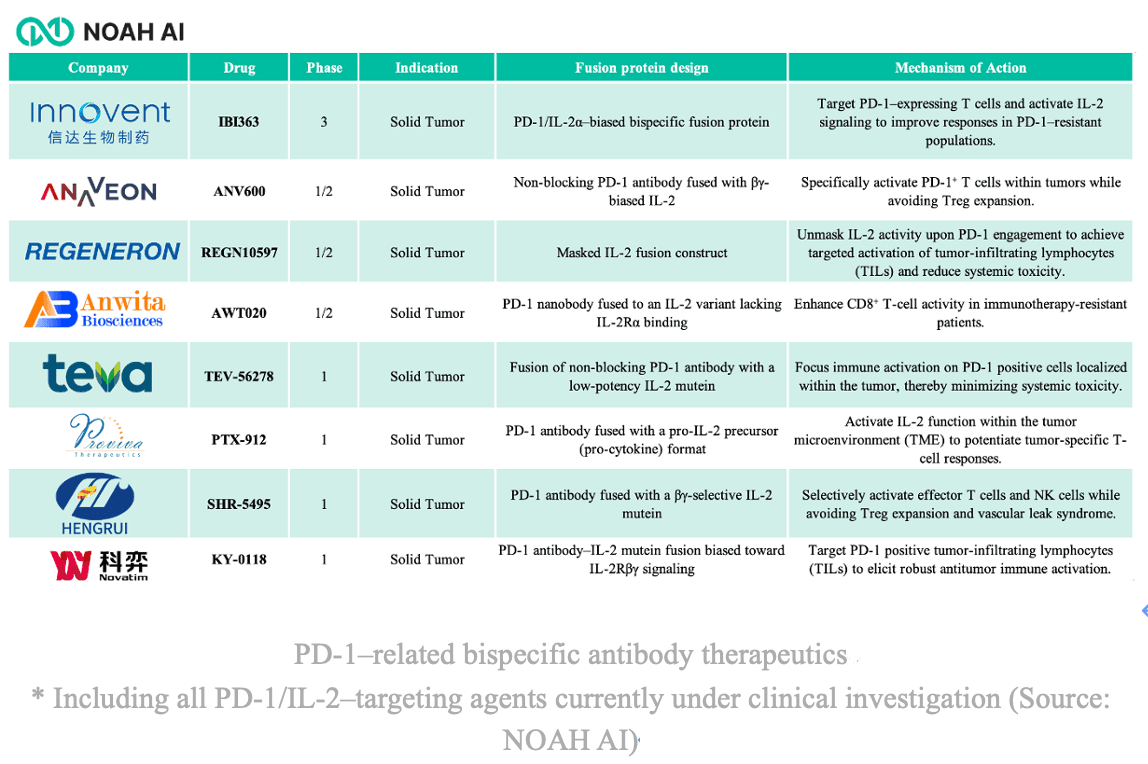
Several global and Chinese biotech companies---including Regeneron, Novatim and Hengrui Pharma --- are pursuing PD-1/IL-2 bispecific programs. Roche's RG6279, which initiate Phase 1 in 2020, was discontinued in July 2025 after limited clinical progress, underscoring the scientific complexity of this approach.

By contrast, Innovent's IBI363 has continued to advance rapidly. Among currently disclosed PD-1/IL-2 pipeline, most remain in early clinical phases, with only IBI363 (Innovent), AWT020 (Anwita), and KY-0118 (Novatim) having released preliminary clinical data showing manageable safety and early signs of efficacy.
4. IBI363: A PD-1/IL-2α Paradigm Born in China
IBI363 stands as the first PD-1/IL-2α bispecific fusion protein to enter multiple Phase 2/3 registration trials globally.
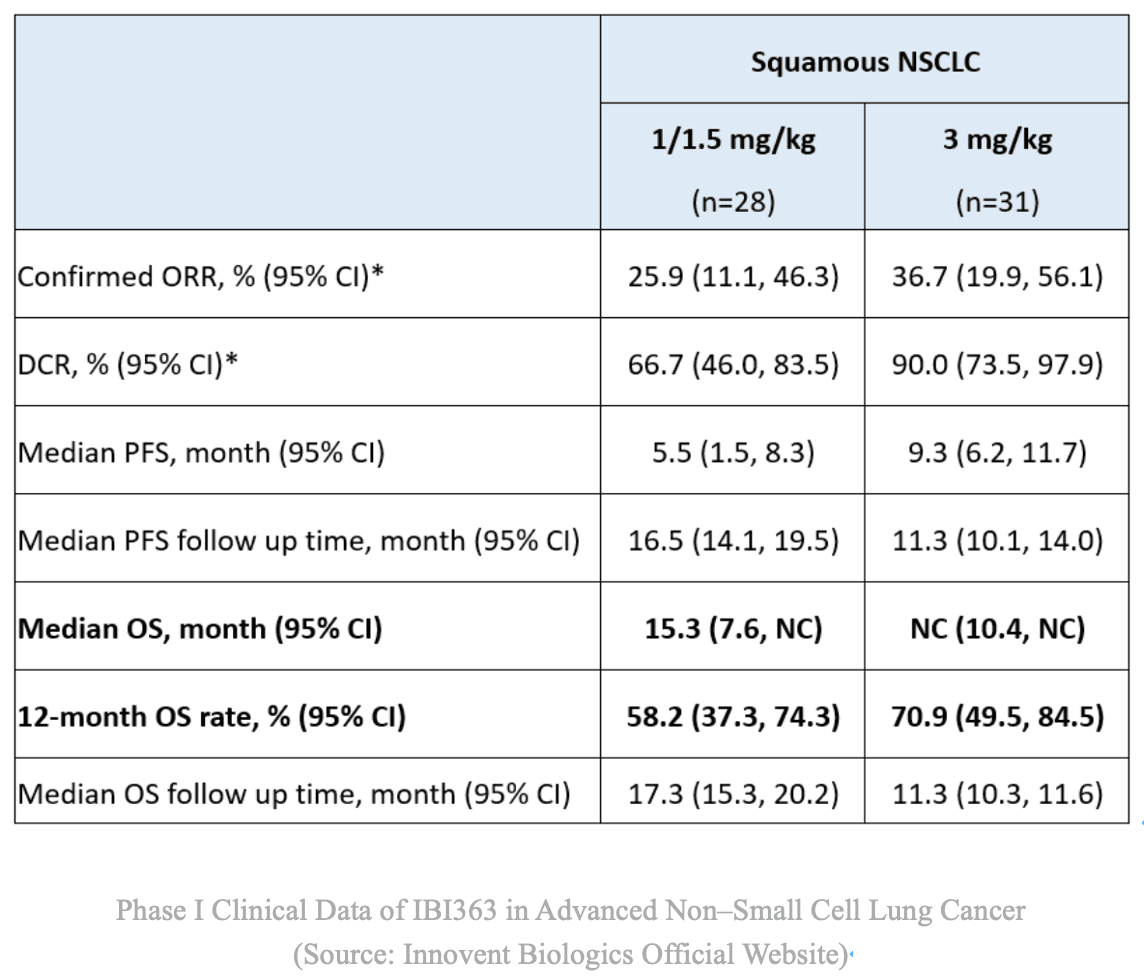
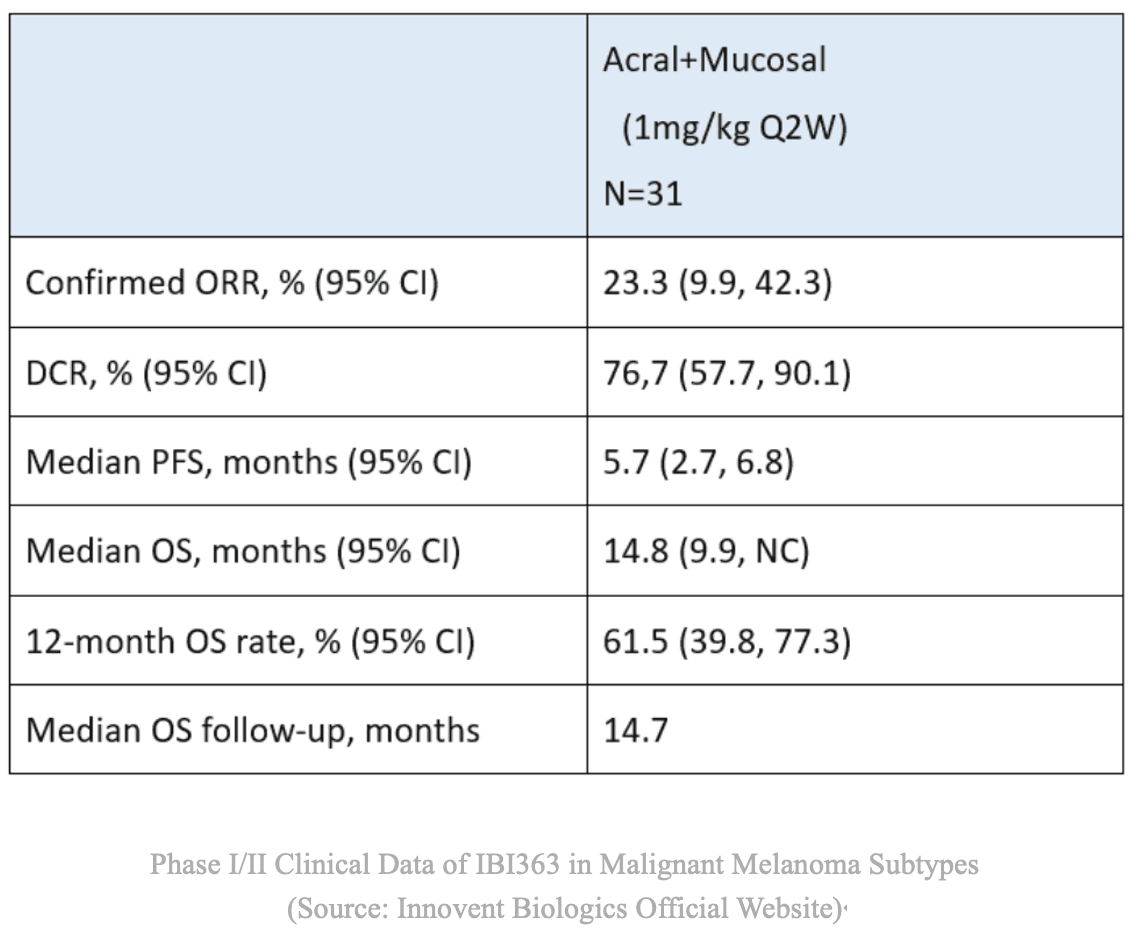
In a Phase 1/2 dose-escalation study involving patients with advanced solid tumors resistant to prior PD-1/PD-L1 therapy, IBI363 monotherapy achieved:
-
Squamous NSCLC (3 mg/kg Q3W cohort): ORR ≈ 36.7%; DCR ≈ 90%.
-
Melanoma (post-PD-1 failure): ORR ≈ 23.3%; DCR ≈ 76.7%.
-
Safety: Overall well-tolerated with manageable adverse events.
Mechanistically, IBI363 is a first-in-class PD-1/IL-2 (α-biased) bispecific fusion protein, designed to block PD-1/PD-L1 while selectively activating the IL-2α receptor. This α-biased design promotes expansion of tumor-specific CD8⁺ T cells while sparing toxicity-associated peripheral T cells, delivering enhanced efficacy with improved safety compared with previous IL-2 therapies.
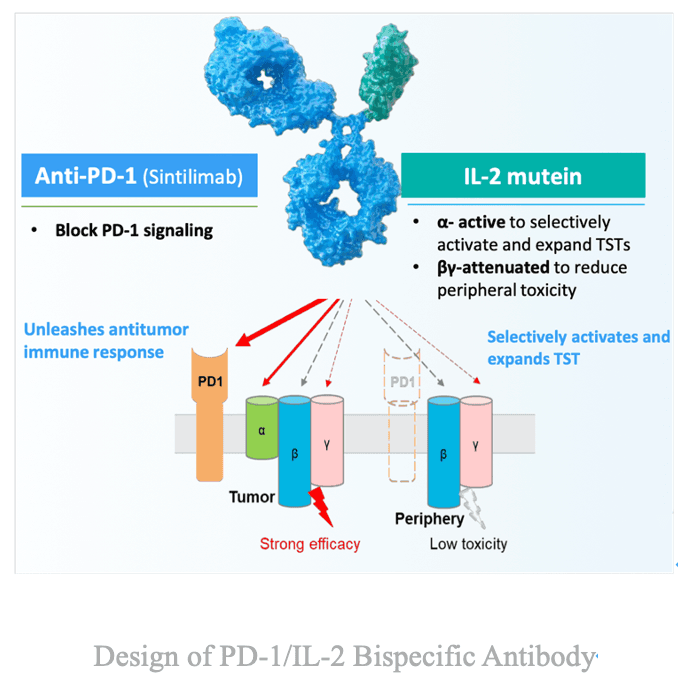
Notably, IBI363 demonstrates responses even in PD-1--resistant populations and initial efficacy signals in immune-cold tumors such as CRC, suggesting the ability to reverse T-cell exhaustion and reinvigorate low-infiltration tumors.
The molecule has received multiple FDA Fast-Track designations and is undergoing global Phase 2/3 trials alongside exploratory Phase 1b/2 combination studies. IBI363 may become the first precision-delivered IL-2 immuno-activator to reach commercialization---marking a critical translational milestone for PD-1/IL-2 bispecific fusion therapeutics.
5. Beyond PD-1/IL-2α: What's Next?
The success of Innovent's IBI363 and other "PD-1 Plus" programs highlight the next frontier in immunotherapy---optimized immune activation, enhanced safety, and synergistic multi-target strategies. Key emerging directions include:
- From PD-1/IL-2α to Multi-Target Synergy: Bispecific fusion proteins such as PD-1/IL-15 and PD-1/IL-21 are emerging to fine-tune immune activation. Most of these programs are currently being developed by Chinese biotech companies in early clinical stages, suggesting significant untapped market potential.
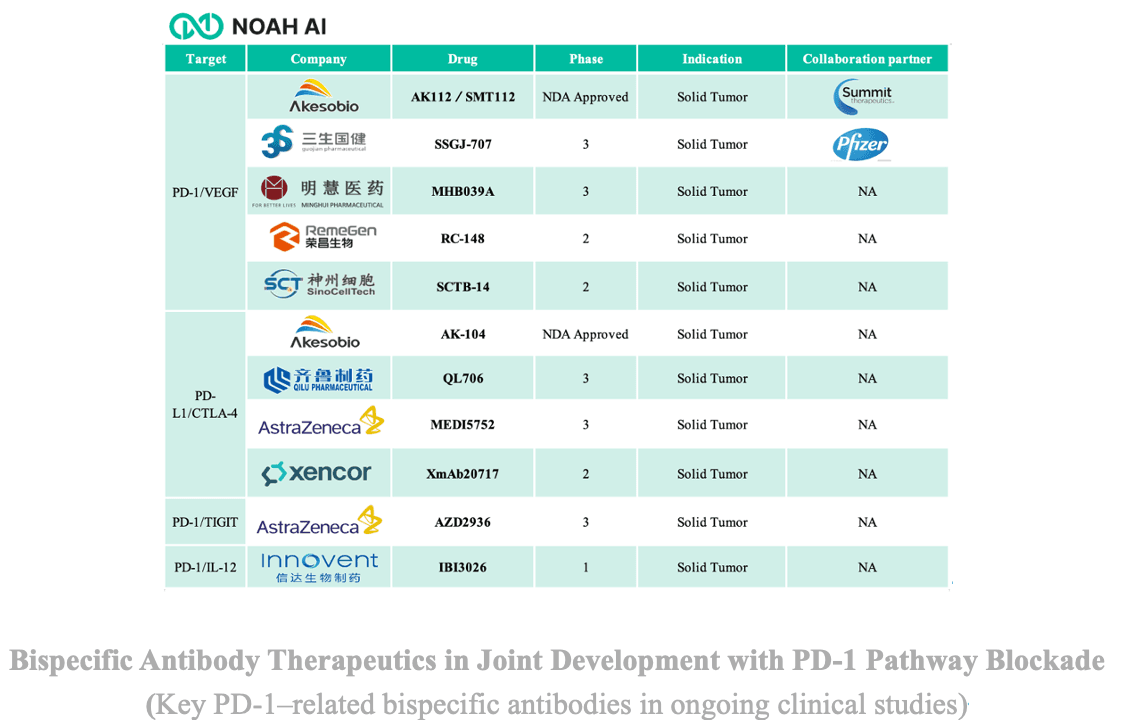
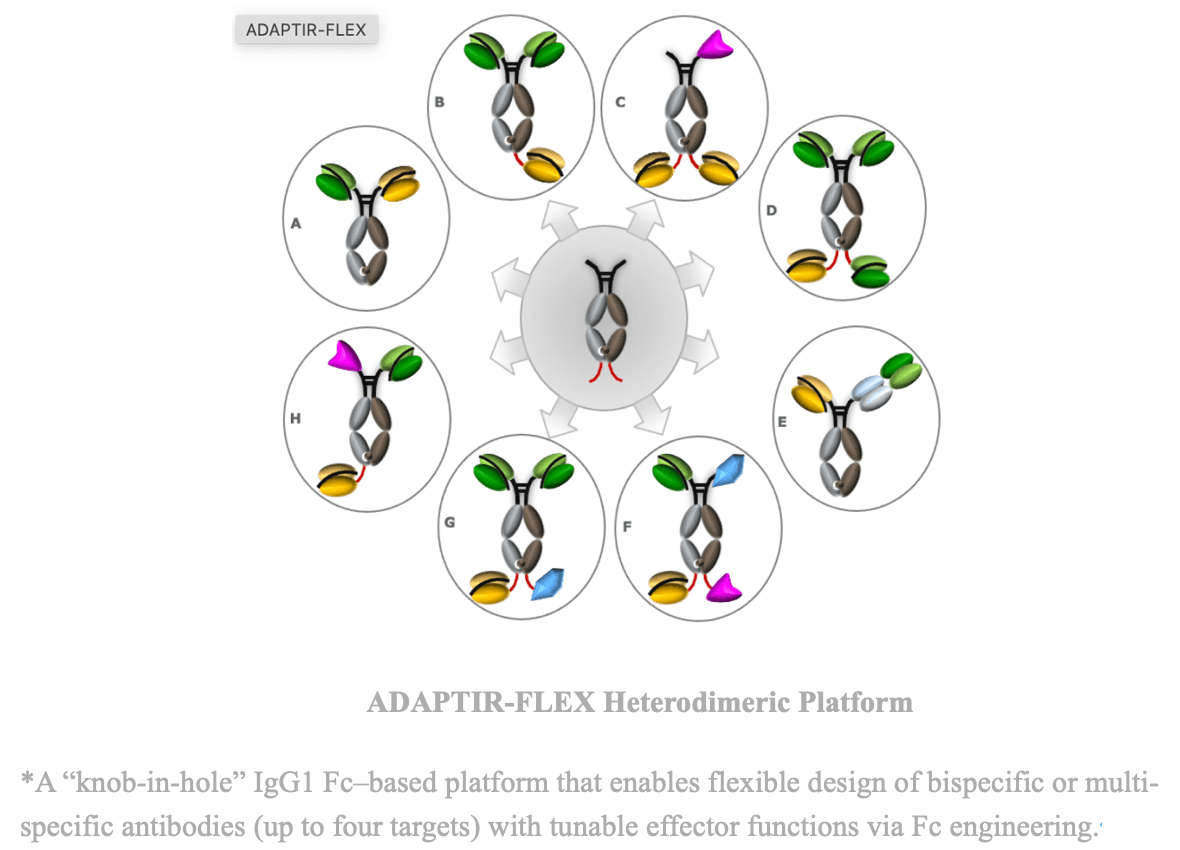
- Molecular Engineering and Structural Optimization: Fc engineering to modulate half-life, immune complex formation, and effector functions, and the use of conditionally activated cytokine designs to restrict activity within the tumor microenvironment.
- Combination Strategies and Indication Expansion: Pairing PD-1 blockade with other inhibitory checkpoints such as CTLA-4 or TIGIT, or combining with metabolic or stromal modulation, to further enhance effector cell function and therapeutic breadth.
6. Conclusion
As the global field advances beyond PD-1 blockade, the Innovent--Takeda collaboration exemplifies a new model of global co-development and technology export from China.
The rise of PD-1/IL-2α bispecific fusion proteins marks a pivotal transition from single-target checkpoint inhibition to precision, multi-dimensional immune system reprogramming. With its strong clinical momentum, IBI363 is poised to become both a first-in-class therapeutic and a driving force behind the paradigm shift in next-generation immuno-oncology.