Introduction of WORKFLOW-Clinical Trial Insights
Comprehensive analysis of drug potential
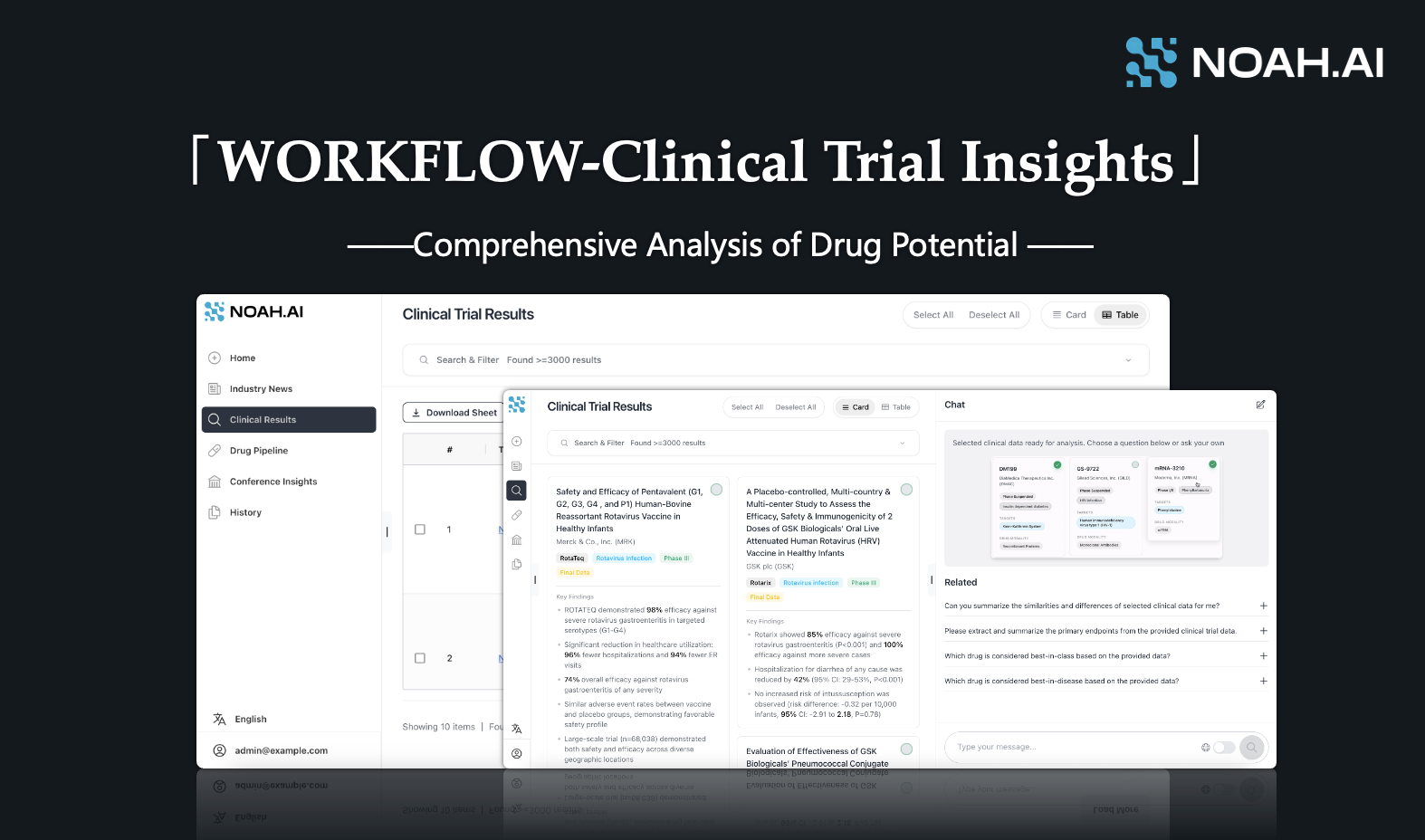
Introduction
A pharmaceutical company's drug pipeline is its core asset. The quality of its clinical data directly determines the value of these assets. Therefore, clinical data analysis is a critical and frequent task for both pharmaceutical professionals and investors.
Tedious and Complex Clinical Data Analysis in Pharmaceutical Pipelines
Pharmaceutical professionals often find themselves spending considerable time gathering, organizing, and analyzing clinical asset data for specific therapeutic areas, particularly when there are no existing reports or external partners to rely on.


Generally, we use ClinicalTrials.gov to search for clinical trials of specific drugs by keywords. However, sometimes we cannot find the relevant data (possibly due to untimely updates). In such cases, we usually need to conduct comprehensive searches using search engines. Ultimately, we often find the target data through literature, conference materials, or news websites.
Regardless of the method, the target data is always embedded in a context filled with extraneous text, making reading and comparison difficult. Therefore, we need to manually extract all the data and summarize and analyze it (e.g., in tabular form). This process is similar to conducting an industry study, where most of the time is spent on data collection and organization.
Introduction of [WORKFLOW-Clinical Trial Insights]
To address the issues above, we have developed the [WORKFLOW - Clinical Trial Insights] feature, aiming to provide users with a more efficient, convenient, and intelligent workflow experience in clinical research analysis.

[Clinical Trial Insights] enables rapid comparison and analysis of clinical trial results, significantly improving efficiency and simplifying complex tasks in the pharmaceutical industry. Key features include Key information pre-processing and AI-powered insights.
Feature Highlights of [WORKFLOW-Clinical Trial Insights]
- Key information pre-processing: Core information is summarized, refined, and presented in a structured format, supporting both card and list views.
- AI-powered insights: Select up to 5 clinical outcomes or 50 drug information for analysis. NOAH AI will interpret and analyze drug potential and its competition landscape.
User Guide and Feature Highlights
To access the [Clinical Trial Insights] feature, you can either start from the homepage or use the sidebar navigation.


To start your research in [Clinical Insights], first filter the relevant clinical trials and data. You can filter by entering at least one of the following: NCT ID, Indication, Drug, Target, Company, Phase, or Location. The corresponding clinical results data will then appear.

Highlight 1-Key information pre-processing
The NOAH AI database integrates all publicly available clinical research data, covering multiple important sources. These include top medical journals (such as The New England Journal of Medicine, Lancet), research findings from internationally renowned medical conferences (such as the American Society of Clinical Oncology), and clinical trial results registered in global medical databases (such as ClinicalTrials.gov, National Institutes of Health, EU Clinical Trials Register, although it currently only supports clinical research searches based on NCT numbers).
In the original clinical data, the information has different entry formats, and the presentation of key numbers also varies, making it impossible for users to directly compare the data in the table. Therefore, NOAH AI extracts and structurally organizes the original data, especially key information such as clinical protocols, effectiveness, and safety, so that users can directly read or even compare the information.
Furthermore, we have included two reading modes to suit different reading preferences: card mode for a quick overview and list mode for detailed reading and comparison.

Card mod: Concise information, presenting only 4-5 key information points (such as key figures for drug efficacy and safety), visually friendly, and suitable for initial screening before in-depth reading.

List mode: Rich information, including detailed information of clinical data such as clinical trial registration number, drug name, indication, target, clinical phase, clinical design, efficacy, safety, source, clinical location, etc., which is suitable for in-depth reading or single-dimensional comparison of various clinical data (such as comparing drug efficacy). In addition, users can also download and export data in Excel format for local processing.

If you need to switch between list mode and card mode, you can just click the button in the upper right corner to switch.

In addition to card mode and list mode, if you need to read the detailed information of a single clinical trial, you can click on the corresponding clinical trial title (as shown in the figure above) to enter the clinical data details page.

The content of the clinical data details page is currently basically the same as the clinical information in list mode, but the interface is more concise, has more reading space, and is more suitable for free operation compared to the table mode. It is suitable for in-depth reading of individual clinical data.
Highlight 2-AI-Powered Insights
After reading the card mode/list mode/details page of clinical data, users may have a general idea of the specific ideas for subsequent research, or which clinical data they need for further research.
In further analysis, the traditional solution is that users need to independently create a table, read, screen, and fill in the core key data, and then perform comprehensive multi-dimensional analysis themselves. The whole process is time-consuming, labor-intensive, and complex. Now, NOAH AI can not only effectively improve the efficiency of user information organization in the early pre-processing of key clinical information, but also directly realize automated analysis in the subsequent data analysis.


Before conducting AI analysis of clinical data, you need to click the middle of the far right side of the browser (as shown in the figure) to summon the AI question box. If you need to engage in immersive reading, you can also click the corresponding button to hide the AI question box, leaving enough space for the core content on the left.

After entering the interface of AI question box, select the target clinical trials (up to 5) in card mode or list mode for NOAH AI to analyze.

Then, enter related questions in the AI question box to ask questions. At present, we will set up some high-frequency questions for clinical data analysis (as shown in the figure above) to facilitate users to click directly. Users can also type personalized questions according to specific business needs to ask questions. Here, in order to ensure the quality of the analysis results, it is recommended that users simplify the questions as much as possible or ask questions in multiple steps.
After submitting the question, the system will immediately call NOAH AI, which is optimized for the medical field, for analysis. NOAH AI will provide professional interpretation of the data on the results page according to the user's question task. After waiting for a while, you can get the analysis results of NOAH AI.
Scenarios of Using WORKFLOW -[Clinical Trial Insights]
NOAH AI's clinical analysis workflow can be applied to the following scenarios:
- Drug Development: Utilize this tool to identify all relevant clinical trials for drugs under development, enabling researchers to gain insights into competitors' R&D progress and clinical data.
- Market Research: Analyze clinical trial results of competing drugs to understand market positioning and adjust product marketing strategies accordingly.
- Clinical Study Design: Employ this tool to locate results from similar clinical trials, optimizing the design of new trials to enhance success rates and data reliability.
- Academic Research: Academic researchers can use this tool to find and analyze clinical trial data in specific fields to support research papers and grant applications.
- Clinical Practice: Physicians can compare inclusion and exclusion criteria across different drugs to identify patient populations with specific outcomes, thereby providing more personalized treatment options.
- Biotech Investment: Investors can leverage this tool to assess the clinical trial results and progress of potential investment projects, analyzing the market potential and risks of drugs to aid investment decisions.
Go to [WORKFLOW -Clinical Trial Insights] for an in-depth exprience.